Pioneering novel approaches to unlock the full potential of IL-1β
Interleukin-1 beta (IL-1β) is a pro-inflammatory cytokine that plays a central role in the pathogenesis of a wide range of human diseases.1 It activates immune cells that generate proinflammatory cytokines, including IL-6, TNF-α, and IL-17. Dysregulated IL-1β signaling is a major driver of inflammation, contributing to the progression of autoimmune disorders.
IL-1β inhibition has proven effective in multiple immune mediated inflammatory diseases.1-3 It also has a well-established class safety and tolerability profile supported by:4-7
- ILARIS (canakinumab), an IL-1β blocker approved in multiple indications
- Data from >7,500 clinical trial participants
- >15 years of real-world experience
Hidradenitis suppurativa (HS) is a chronic, progressive inflammatory skin disease that causes painful nodules, abscesses, and tunnels to be formed under the skin.1-4 If not adequately and promptly treated, the chronic inflammation characteristic of HS may progress to tissue destruction and permanent scarring.1-3,5
There is a substantial unmet need for more effective and durable treatment options
Currently approved biologics for HS (adalimumab [anti-TNF-α], secukinumab [anti-IL-17A], and bimekizumab [dual anti-IL-17A/F]) achieve HiSCR50 (an efficacy measure) only ~50% of the time, with more stringent thresholds (HiSCR75/90) achieved in even fewer individuals.6-9 Long-term disease control remains a challenge with established biologics and an area of uncertainty with newer agents (secukinumab and bimekizumab), as data on sustained efficacy are still emerging.6-9 In addition, people with HS often have comorbidities that can limit access to current treatment options.
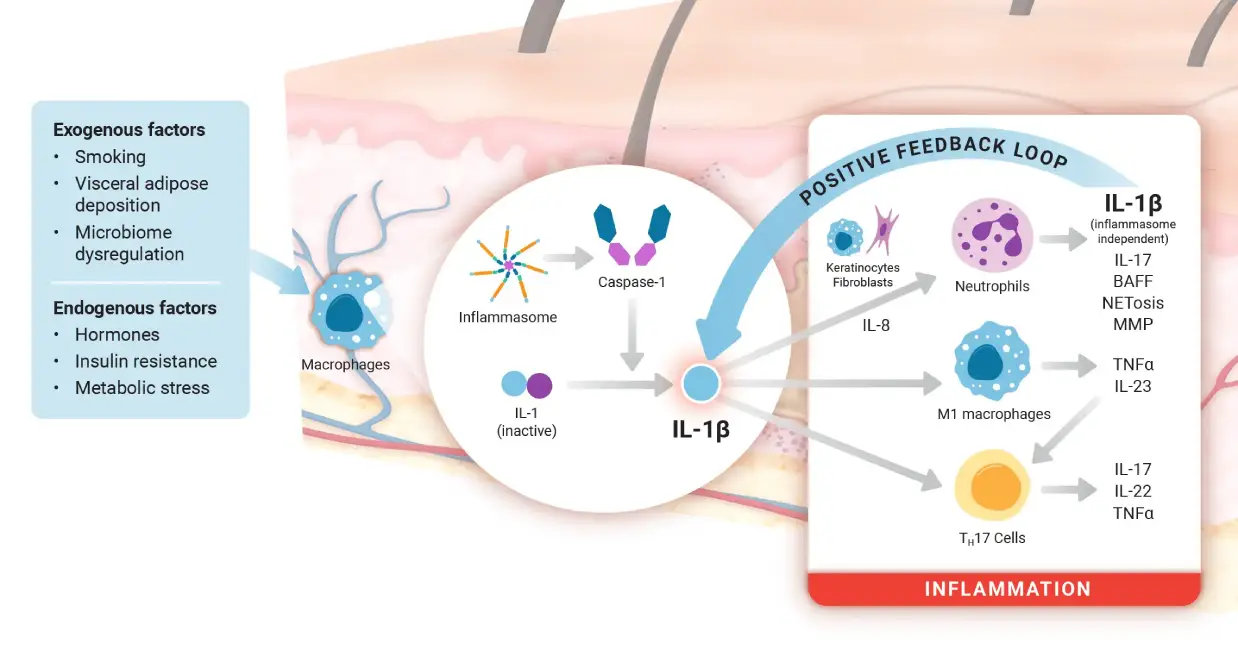
IL-Iβ dominates the pathophysiology of HS2,10,11
Key clinical evidence supporting IL-1 inhibition as an approach to treating HS
In a Phase 2 trial with lutikizumab (an IL-1α/β bispecific), participants receiving lutikizumab achieved a treatment effect comparable to other HS therapies, despite the fact that the trial evaluated a more severe patient population.12 In addition, MAS825 (an IL-1β/IL-18 bispecific) showed positive results in a Phase 2 randomized controlled study in HS.13-16

